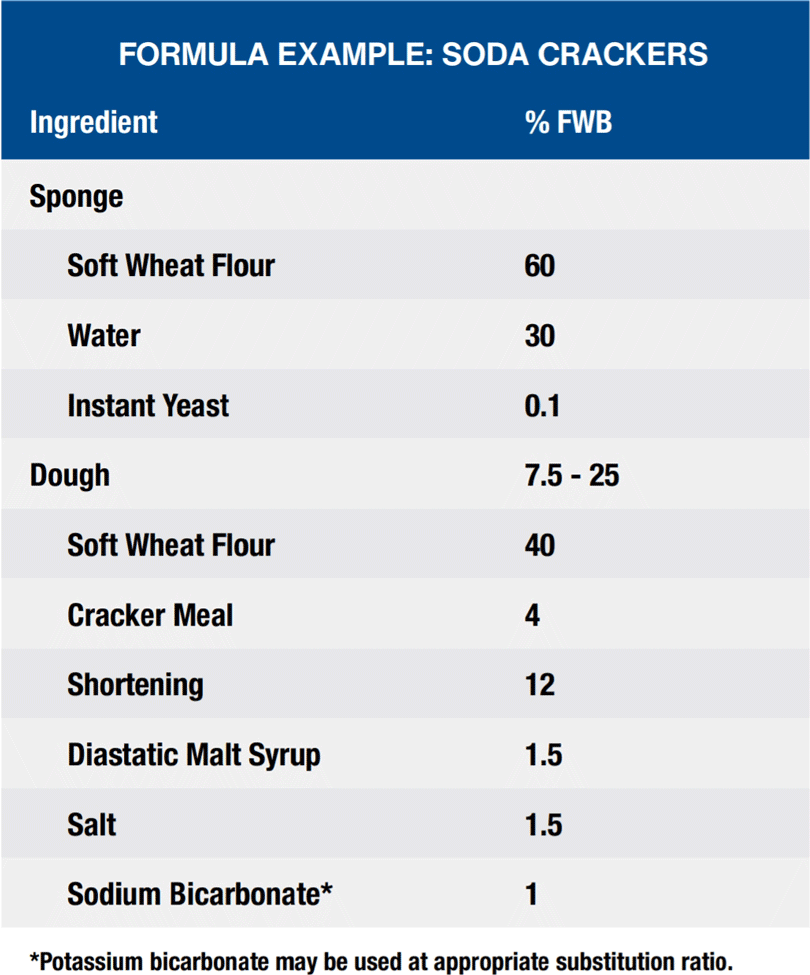
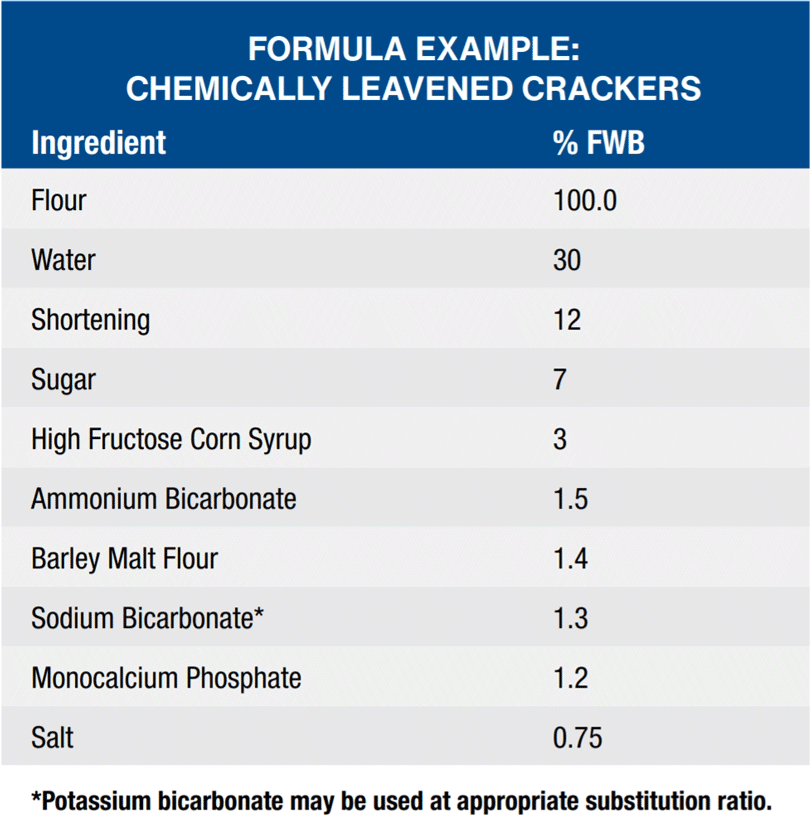
Crackers – Bicarbonate Applications
Snack crackers encompass a broad spectrum of products ranging from semi-sweet, machine-cut, chemically leavened cookie-like crackers to non-sweet, fermented, laminated crackers. In general, crackers contain little or no sugar, moderate levels of fat, and relatively low levels of water. Crackers can be divided into two basic categories: soda crackers (saltines) and snack crackers (sprayed and savory). A third group consists of graham crackers which are made with higher levels of sugar. Leavening can be accomplished either by yeast fermentation or by chemical leavening. Most yeast leavened crackers (saltines) are processed using a sponge and dough fermentation process. The bicarbonates serve to neutralize the acids formed from the fermentation reaction. Chemically leavened crackers are processed utilizing the carbon dioxide produced from the reaction of the bicarbonate with an acidic salt.
Sodium, potassium and ammonium bicarbonate function as leaveners to provide gas release which results in the rise of the cracker. Cracker height and texture are dependent on the use of bicarbonates for proper leavening. Bicarbonates also function to control the pH of the system which impacts the flavor and color of the cracker.
Bicarbonate Recommendation
Sodium Bicarbonate Grade 1 Powdered:
Dissolves rapidly to assure quick, complete availability for reaction with the acid ingredients.
Sodium Bicarbonate Grade 1 TFF:
Treated with tricalcium phosphate to improve flow quality. Dissolves rapidly to assure quick, complete availability for reaction with acid ingredients.
Sodium Bicarbonate Grade 2 Fine Granular:
The narrow particle size distribution of Grade 2 facilitates rapid, uniform blending. This grade is recommended for those products where minimal leavening during mixing and holding is desired. It is also recommended for products where pre-reaction during storage can be a problem.
Flow K™ Potassium Bicarbonate:
Potassium bicarbonate performs exceptionally well as a replacement for sodium bicarbonate in most chemically leavened products. For equivalent CO2 release, 19% more potassium bicarbonate must be used.
Ammonium Bicarbonate:
Reacts rapidly in the presence of moisture and/or heat to release CO2 and NH3 gases which contribute to leavening. Use of ABC without a leavening acid is limited to products whose final moisture is < 5% so the ammonia gas can bake out.